Get A Quote
Testimonials
Enough about us. Let’s have our customers talk about us.
- mohamed lahlou, sonovascular
Working with GII has been a great experience—their team is highly responsive and always willing to provide thoughtful, knowledgeable feedback. They've consistently collaborated with us to refine our product design and specifications, ensuring everything aligns with our evolving requirements.
- william prentice, co-founder and ceo of signati medical
Global Interconnect has been a great partner for us. They have been there every step of the way for us, to advise us in other spots that they don’t cover. Picking the right partner, and making sure we spend our money wisely, that’s the big key.
- project engineering manager, early stage women's health oem
It has been excellent working with you and I have been really impressed by the work carried out by the team to get us towards where we need to be on the cable cosmetics. It feels like we have made such good progress and are very close. Thank you so much for the ongoing support.
- buyer, leading lifescience oem of surgical devices
Cheryl and the team are always so helpful and always come back with any queries we have.
White Paper
Explore How Value Engineering Transforms Medical Device Manufacturing
Accelerate MedTech development through optimized design, cost-effective production, and faster product launches.
See how Global Interconnect leverages Value Engineering (VE) to improve medical device manufacturing—boosting product performance, ensuring regulatory compliance, and supporting long-term innovation. This white paper details how VE simplifies design, optimizes materials, streamlines processes, and fosters stronger supplier partnerships to deliver meaningful results.
Blog
Connective thinking from Gii.

Global Interconnect’s Director of Quality Enhances Product Quality With Asia Visit
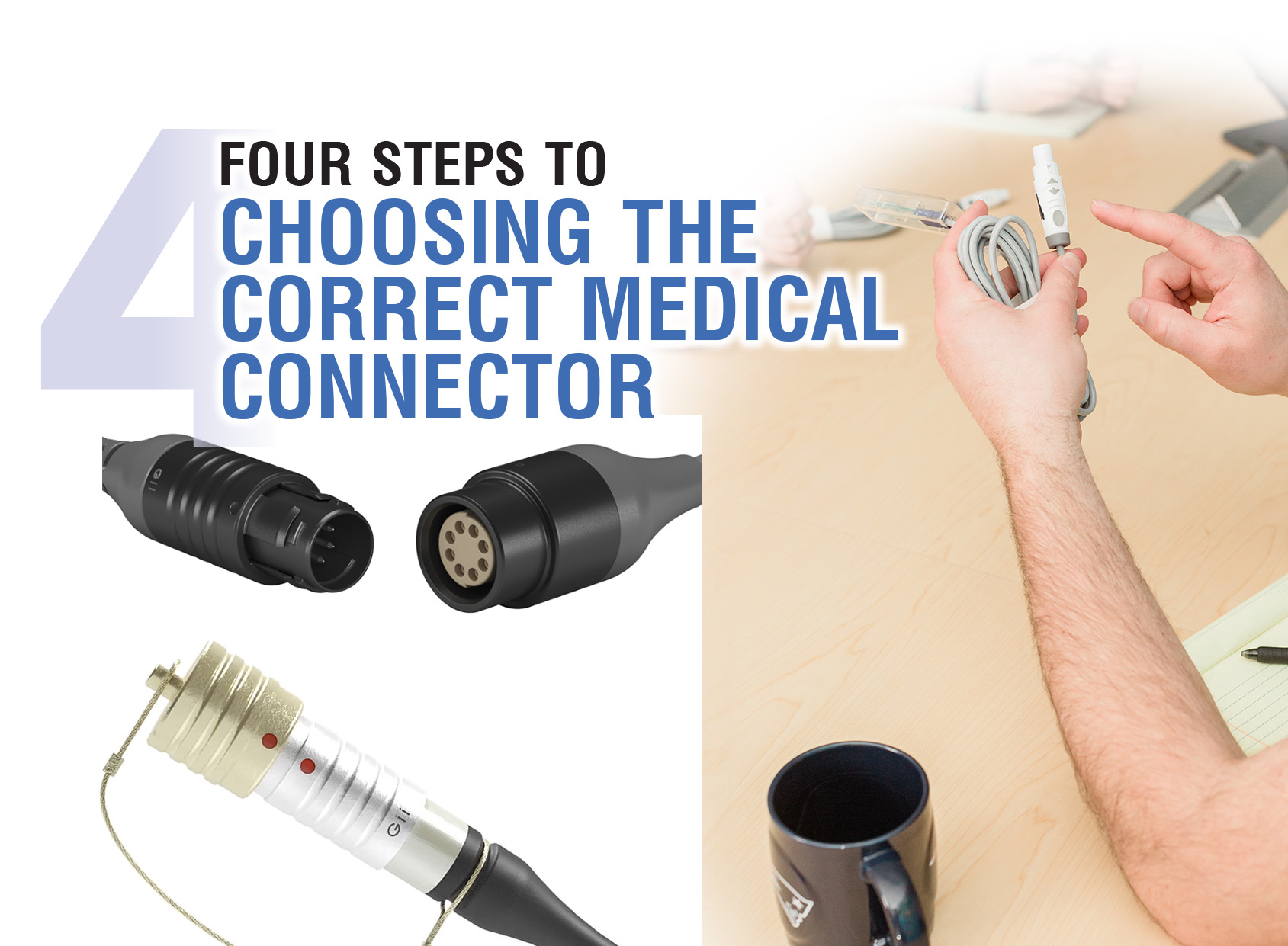
4 Steps to Choosing the Correct Medical Connector

Design Considerations for Reusable Medical Connectors

How Global Interconnect Changed the Game for a Global Sports Medical Device Manufacturer.

Global Interconnect Partners with Materialogic to Provide Enhanced Logistics to their Medical Device Customers

The Quarterly Connection – Q1 2023

Design Considerations Single-Use Medical Connectors
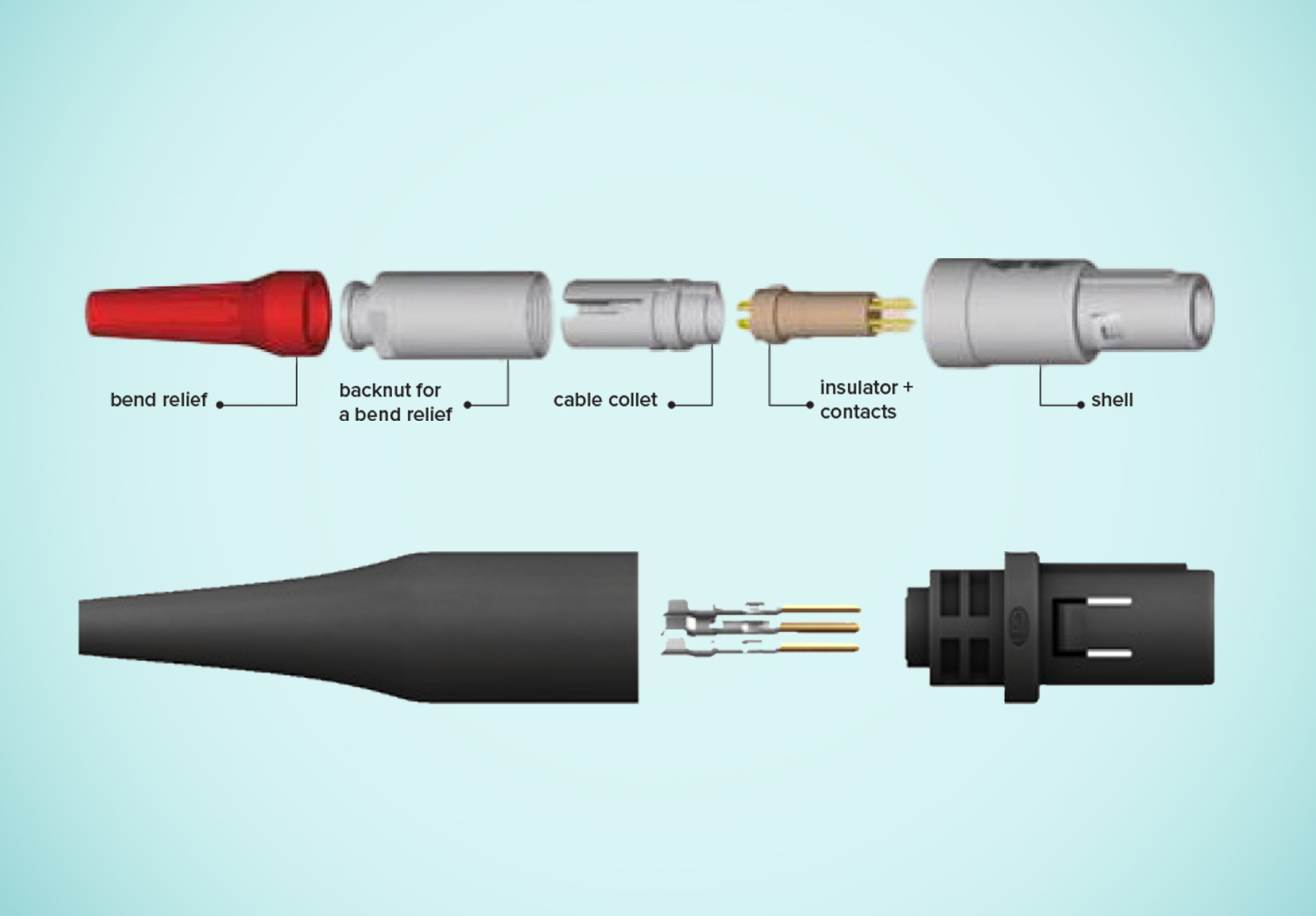
Radio Frequency (RF) Ablation Connector: Improve Cost and Performance
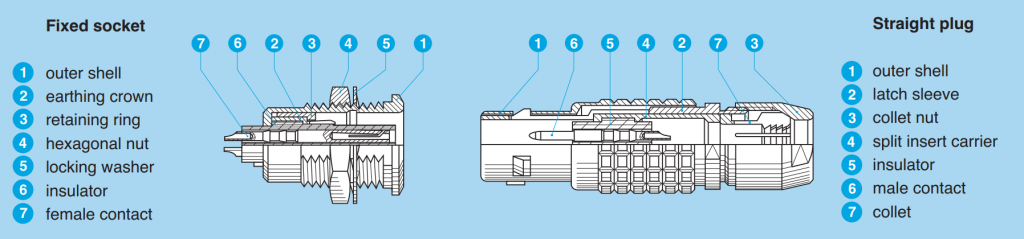
Understanding Lemo and Fischer Push Pull Connectors

Custom Components and Assemblies

Learning Custom Medical Cable Assembly Materials

Understanding Medical Cable Assemblies

A Message from our CEO: The Benefits of our Model

Supply Chain Management During COVID-19

Understanding Custom Medical Connectors
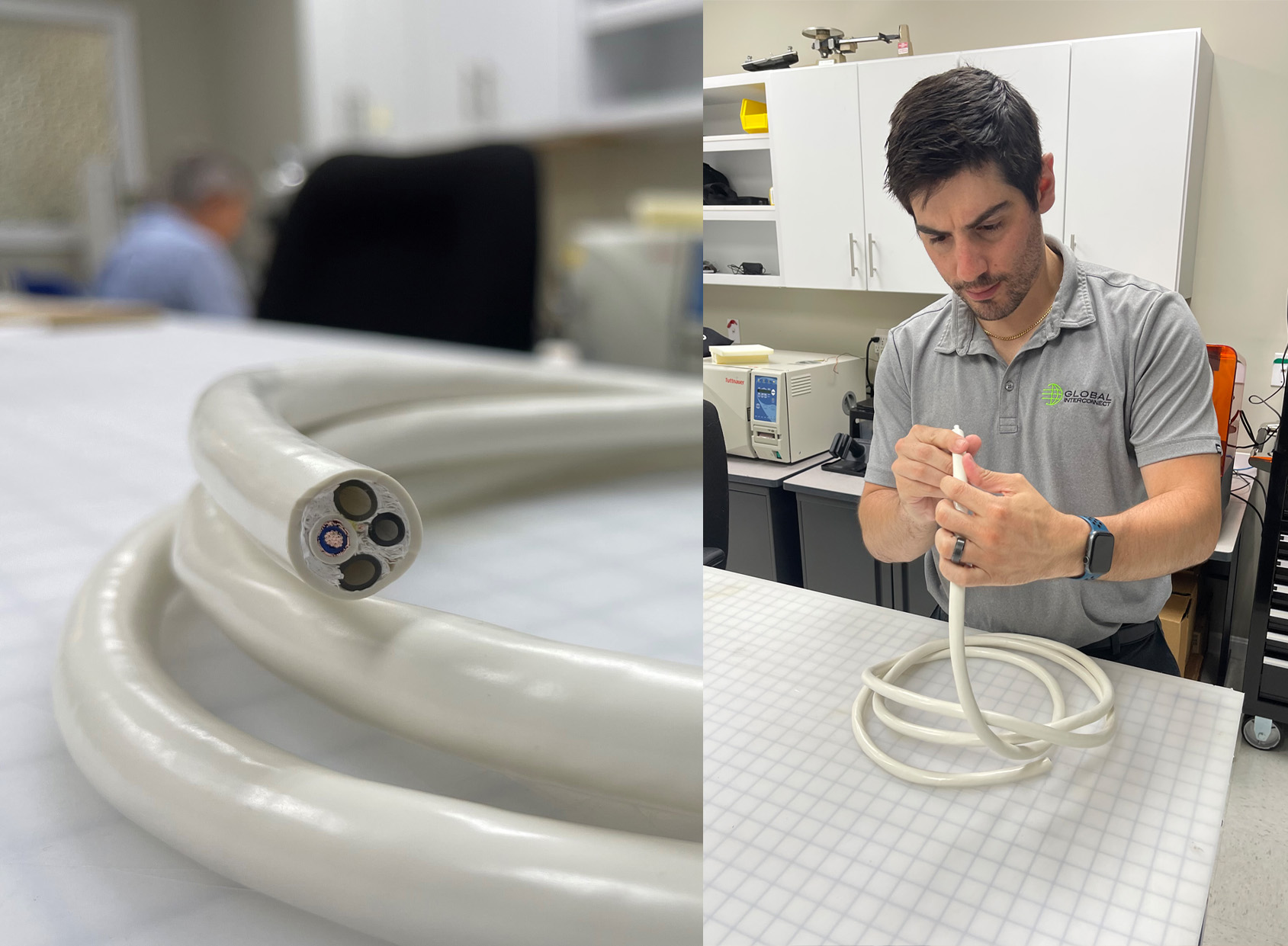
Braided Cable Sleeve vs. Extruded Cables

Human Factors and Medical Devices
