Get A Quote
Capabilities
With global partnerships that allow us access to over 10,000 workers, 1.5 million square feet of manufacturing capacity, 15,000 square feet of clean room capacity, and eight sterilization chambers totaling 10,000 cubic feet, we can support the world’s largest medical device manufactures and build tens of millions of devices annually.
Sub-Assembly
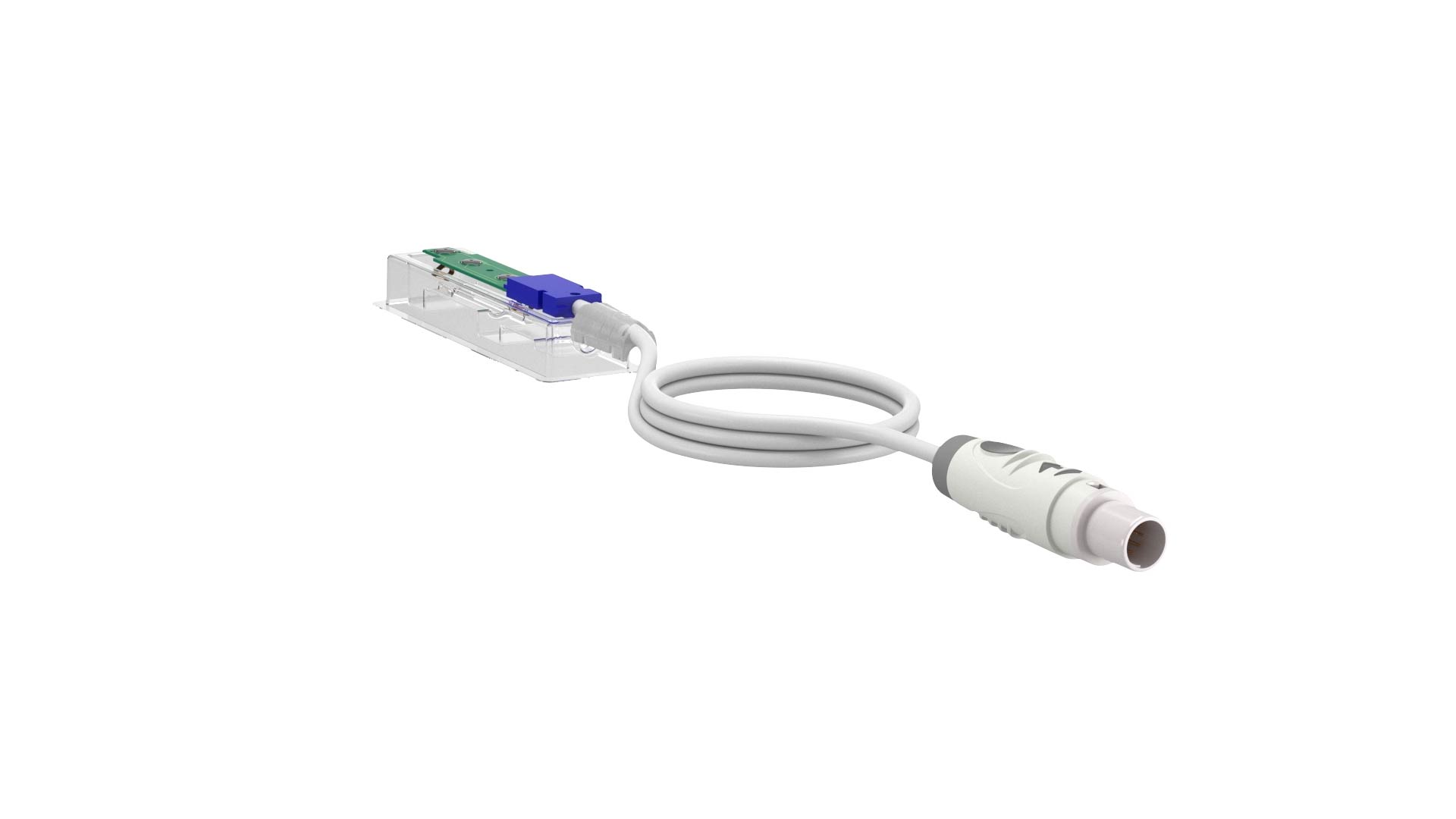
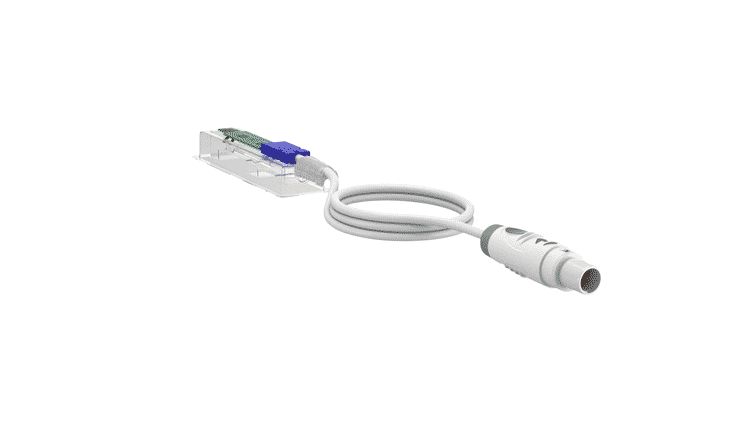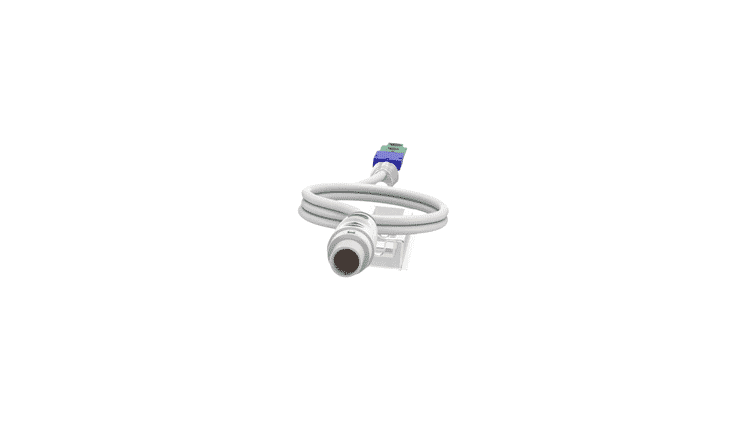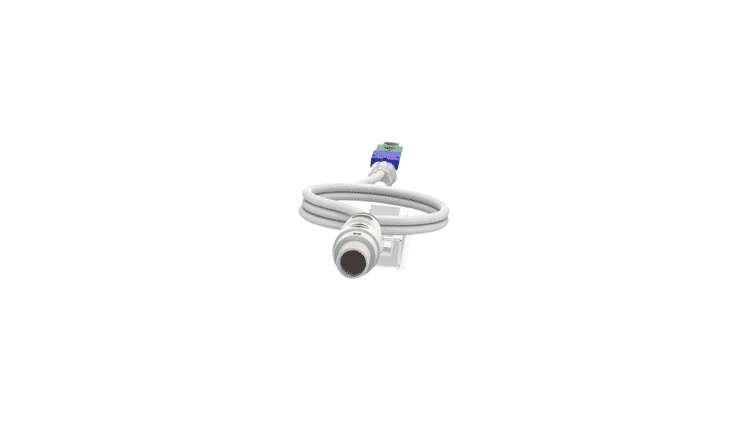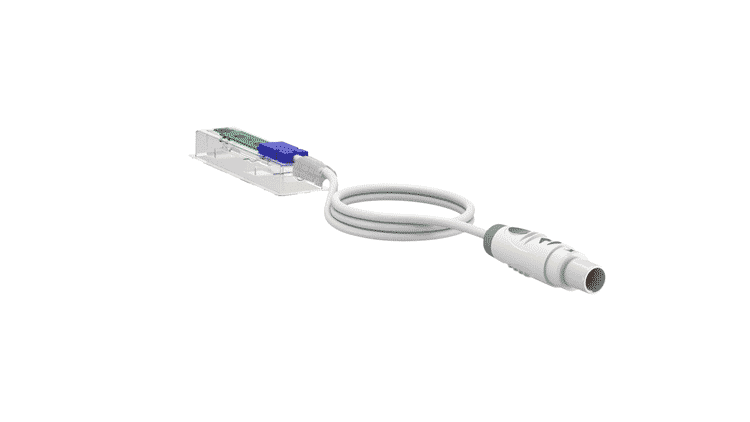
Finished Medical Device


Sterilization
Sterilization
Gii excels in designing and manufacturing sterilizable reusable and single-use medical devices.
Cleanroom
Packaging
Assembly
Tooling
Molding
Extrusion
Machining
Coating
Coating
Gii uses parylene coatings for superior moisture, chemical resistance, and low friction.